how to draw molecular orbital diagram of no
O is more electronegative than N so its orbitals are slightly lower in energy and the bonding orbitals. Molecular Orbital MO Theory is the final theory pertaining to the bonding between molecules.
How Do We Draw The Molecular Orbital Diagram Of Bf Quora
The applications of the MO theory extend beyond the limitations of the Valence Shell Electron Pair Repulsion VSEPR model and the Valence Bond theory.

. See Resources for a diagram showing the filling order. These are directed towards the four corners of a regular tetrahedron and make an angle of 10928 with one another. Bond order can be calculated by the formula.
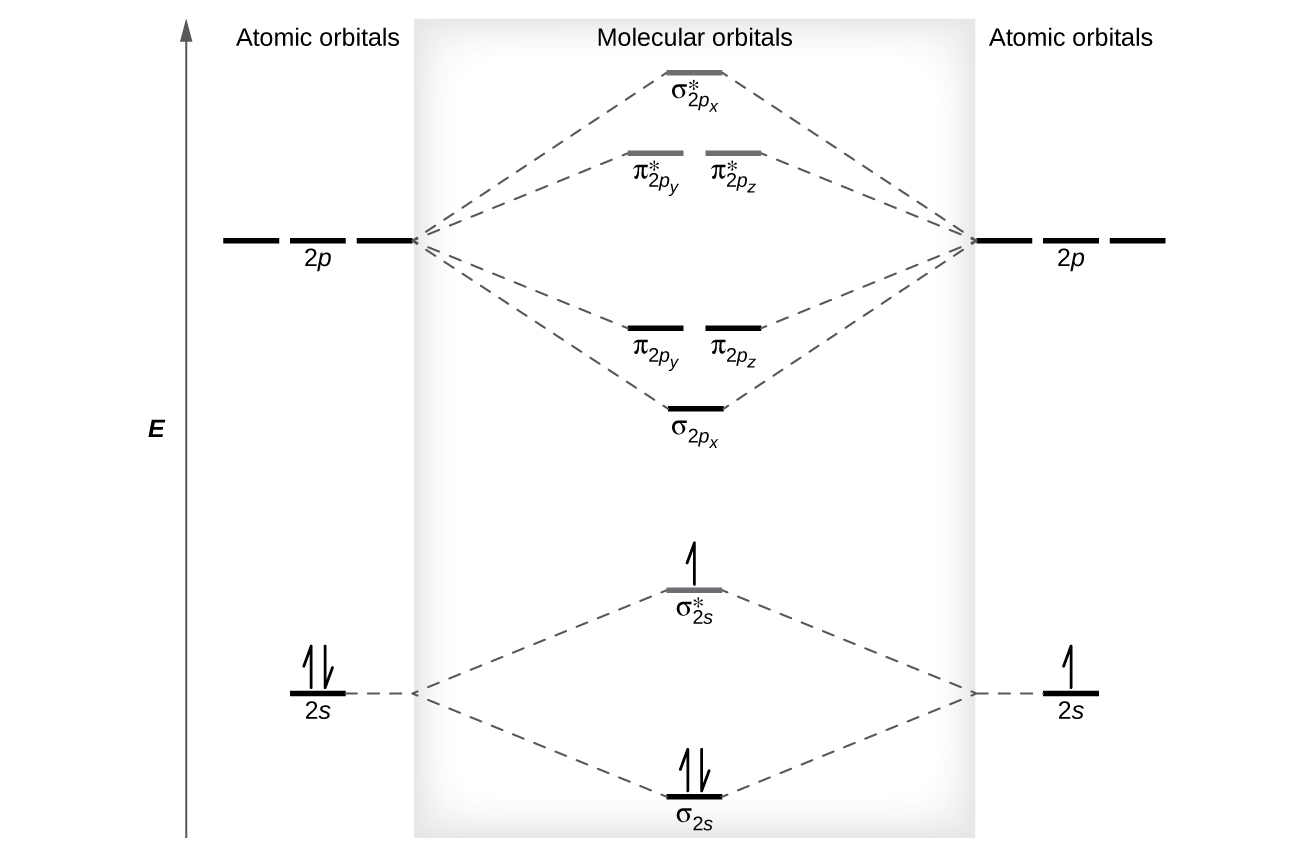
σ1s. Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. 8 - Drawing Molecular Orbital Diagrams Flux Science.
8 - Drawing Molecular Orbital Diagrams. The molecular orbital diagram for Nitrogen dioxide NO₂ should loo. Procedure to draw the molecular orbital diagram of CN.
Depending on if it is a homonuclear case where the bonding atoms are the same or a. Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow. Example of sp 3 hybridization.
Fill the electrons in empty boxes using three principles Aufbau Hunds and Pauli Exclusion. Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. I think you can safely assume to start off with the molecular orbital diagram of the Nitrite anion NO₂ and then remove an electron from it.
First week only 499. Number of electrons in antibonding orbitals. Molecular orbital diagram of N 2 is shown below.
I was just wondering if the same applied for molecules with a. The Aufbau principle tells you that the lowest-energy orbitals fill first but the specific order isnt sequential in a way thats easy to memorize. Parallel 2p orbitals interact strongly with one another no matter how many of them are present.
So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each. Each horizontal line represents one orbital that can hold two electrons. This picture shows the molecular orbital diagram of N 2.
Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. BO 12 bonding e- - antibonding e- 122222 - 21 colorblue25 And this should make sense because NO is isoelectronic with CO which has a bond order of 3. Draw and explain all the properties of CO and NO with the help of molecular orbital diagram.
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. As was true in forming sigma and pi molecular orbitals the number of 2p orbitals that are interacting is the same as the number of molecular orbitals that are formed. Note that the n 1 level only has s orbitals the n 2 level only has s and p orbitals and the n 3 level only has s p and d orbitals.
MAKE SURE TO SUBSCRIBEThis video puts emphasis on molecular orbital diagrams a fundamental way of understanding why Diels-Alder chemistry works. In the link above chem_mod said it was best to account for the negative charge of CN- by placing an extra electron on the nitrogen since it is more electronegative. Ethane C 2 H 6 methane.
To draw the orbital diagram for an atom follow these basic steps-. So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. Clearly carbon has 4 valence electrons and nitrogen has 5.
Orbitals represented by are antibonding orbitals and the orbitals without are bonding orbitals. These previous theories provide substantial. Lets take an example of the Nitrogen atom.
Same as NO but change 1 at the end to 2. Find the valence electron of each atom in the CN molecule. Molecular orbital theory diagram for Br2.
Bond order bonding electrons - antibonding electrons 2. 2 So the formula to find bond order is Bond order dfrac12 Number of electrons in BMO Number of electrons in ABMO Bond order. Find the number of electrons in an atom.
Sp 3 Hybridization The new orbitals formed are called sp 3 hybrid orbitals. Start your trial now. We will develop this topic more when we discuss concerted chemical reactions.
The molecular orbital MO theory is a powerful and extensive approach which describes electrons as delocalized moieties over adjacent atoms. In contrast to VSEPR and valence bond theory which describe bonding in terms of atomic orbitals molecular orbital theory visualizes bonding in relation to molecular orbitals which are orbitals that surround the entire molecule. To obtain the bond order look at the molecular orbitals formed and decide whether they are bonding or antibonding.
Procedure to draw the molecular orbital diagram of CN. When considering bonding between two atoms first construct the relevant molecular orbitals then fill them with the available electrons starting with the lowest energy molecular orbital. Next well see that symmetry will help us treat larger.
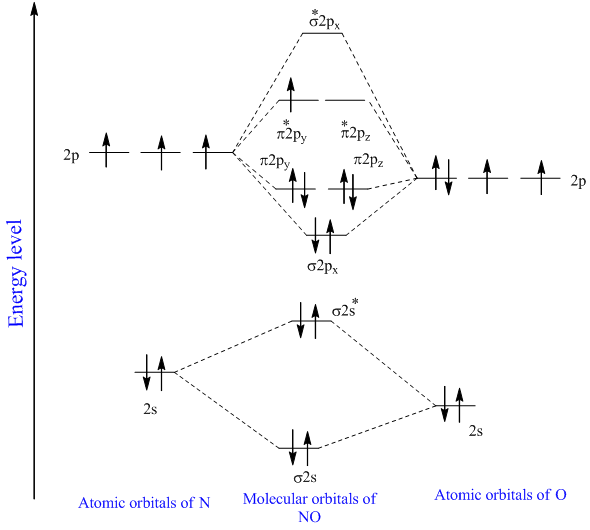
Your MO diagram for NO should look like this. For a diatomic molecule the atomic orbitals of one atom are shown on the left and those of the other atom are shown on the right. Each sp 3 hybrid orbital has 25 s character and 75 p character.
2 So the formula to find bond order is Bond order dfrac12 Number of electrons in BMO Number of electrons in ABMO Bond order dfrac12 8 2 Bond order dfrac12 6 Bond order 3 - N_2 molecules are diamagnetic with no unpaired electrons. The purpose of MO theory is to fill in the gap for some. Solution for Draw the molecular orbital diagram for NO3-close.
Compare the bond order to that seen in the Lewis structure remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a. What will be the molecular orbital diagram for nitrite ion. Photoelectron spectroscopy provides useful information on the energies of atomic orbitals.
Write the electron configuration for an atom to determine which orbitals should be filled. Clearly CN is hetero orbital. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram.
Molecular Orbital Diagram of NO. Construct a qualitative molecular orbital diagram for chlorine Cl 2.
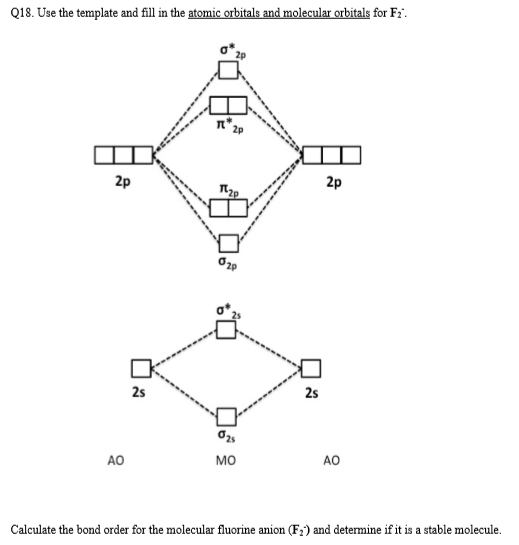
Solved Q20 Draw The Molecular Orbital Diagram For The Chegg Com
Explain The Mo Diagram For No Molecule Sarthaks Econnect Largest Online Education Community
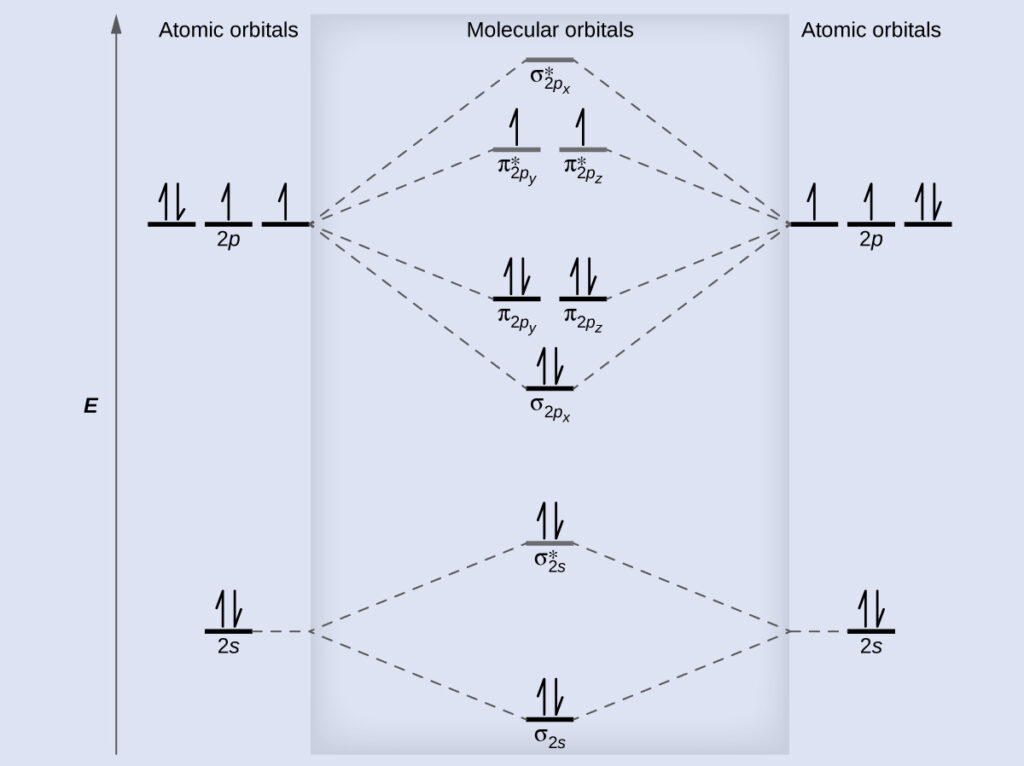
Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Chem Textbook
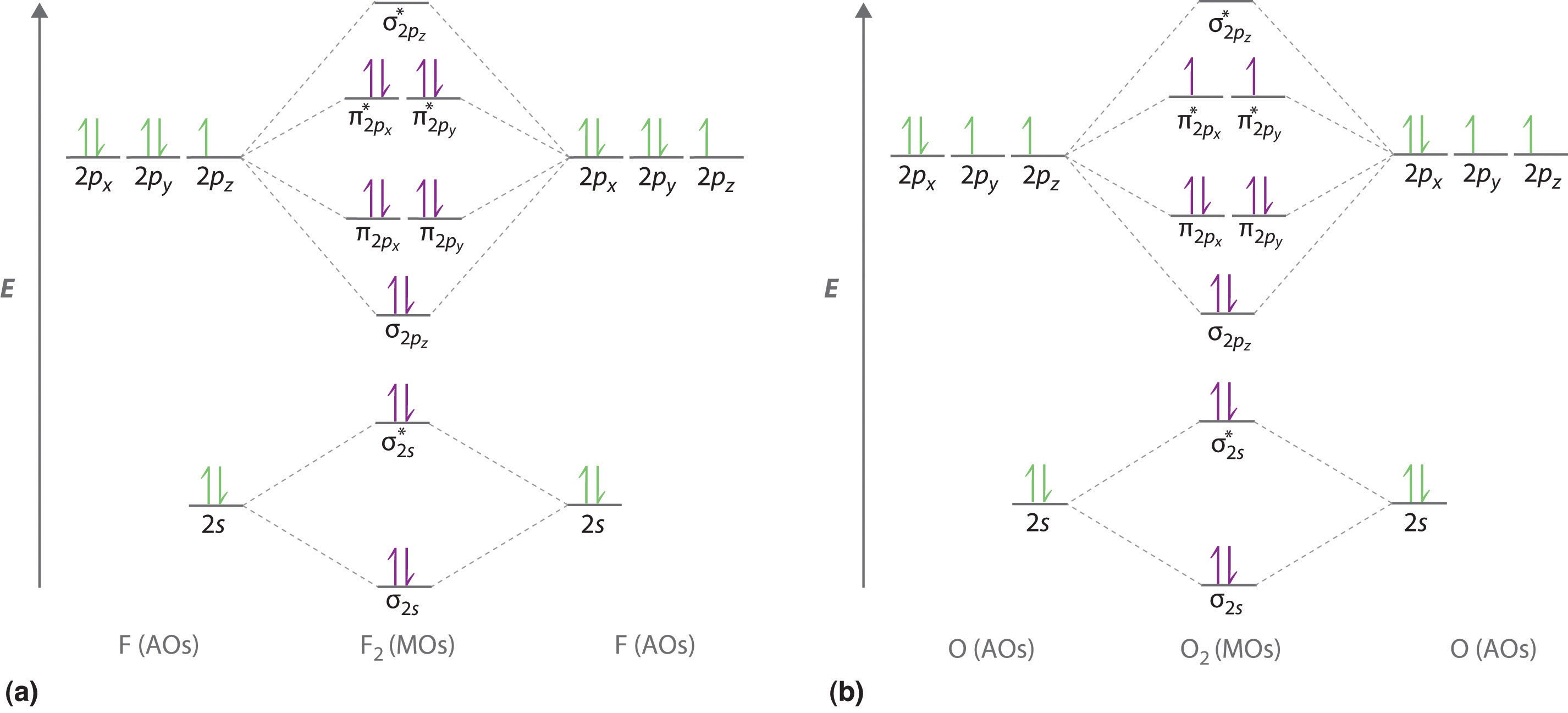
2 6 Molecular Orbital Theory Chemistry Libretexts
Solved Chapter 5 Problem 7p Solution Inorganic Chemistry 5th Edition Chegg Com

Delocalized Bonding And Molecular Orbitals

By Writing Molecular Orbital Configuration For No Co O2 Molecules Calculate The Bond Order And Also Determine Whether It Is Paramagnetic Or Diamagnetic Socratic
What Is The Molecular Orbital Diagram For No Quora

Mo Diagram Overview How To Draw Mo Diagram And Solved Example Along With Faqs

Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com

File Nitric Oxide Mo Diagram Svg Wikimedia Commons
2 2 Molecular Orbital Mo Theory Review Chemistry Libretexts

Molecular Orbital Diagram For No Download Scientific Diagram

A Calculate And Display The Molecular Orbitals Of No Show How The Reaction Of No And H Can Be Described As A Homo Lumo Interaction B Calculate And Display The Molecular Orbitals Of

Mathematics Origins Of Molecular Orbital Diagrams History Of Science And Mathematics Stack Exchange

Construct Molecular Orbital Diagram And Determine Unpaired Electrons In O2 O2 Bn No Study Com

Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack Exchange